


 Products
ProductsPeppermint is an organic compound made synthetically or obtained from cornmint, peppermint or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation. Menthol also acts as a weak kappa opioid receptor agonist.
Structure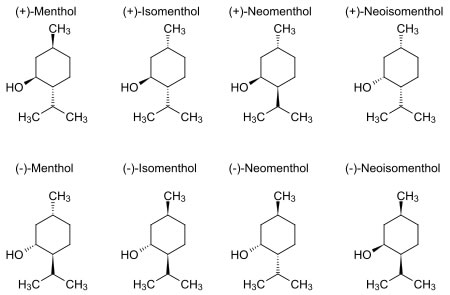
In the natural compound, the isopropyl group is in the trans orientation to both the methyl and hydroxyl groups. Thus, it can be drawn in any of the ways shown: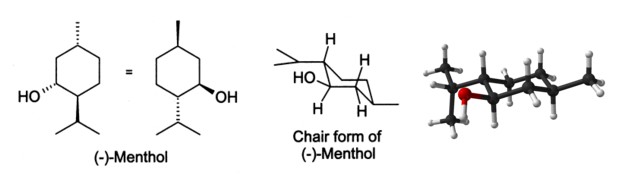
Biological properties
Menthol's ability to chemically trigger the cold-sensitive TRPM8 receptors in the skin is responsible for the well-known cooling sensation it provokes when inhaled, eaten, or applied to the skin.In this sense, it is similar to capsaicin, the chemical responsible for the spiciness of hot chilis (which stimulates heat sensors, also without causing an actual change in temperature).
Menthol's analgesic properties are mediated through a selective activation of κ-opioid receptors.Menthol also blocks voltage-sensitive sodium channels, reducing neural activity that may stimulate muscles.Menthol also enhances the efficacy of ibuprofen in topical applications via vasodilation, which reduces skin barrier function.
Occurrence
(−)-Menthol (also called l-menthol or (1R,2S,5R)-menthol) occurs naturally in peppermint oil (along with a little menthone, the ester menthyl acetate and other compounds), obtained from Mentha x piperita.Japanese menthol also contains a small percentage of the 1-epimer, (+)-neomenthol.
Biosynthesis
Biosynthesis of menthol was investigated in M. x piperita, and all enzymes involved in its biosynthesis have been identified and characterized.[6]
More specifically, the biosynthesis of (−)-menthol takes place in the secretory gland cells of the peppermint plant. Geranyldiphosphate synthase (GPPS), first catalyzes the reaction of IPP and DMAPP into geranyldiphosphate. Next (−)-limonene synthase (LS) catalyzes the cyclization of geranyldiphosphate to (−)-limonene. (−)-Limonene-3-hydroxylase (L3OH), using O2 and NADPH, then catalyzes the allylic hydroxylation of (−)-limonene at the 3 position to (−)-trans-isopiperitenol. (−)-Trans-isopiperitenol dehydrogenase (iPD) further oxidizes the hydroxy group on the 3 position using NAD+ to make (−)-isopiperitenone. (−)-Isopiperitenonereductase (iPR) then reduces the double bond between carbons 1 and 2 using NADPH to form (+)-cis-isopulegone. (+)-Cis-isopulegoneisomerase (iPI) then isomerizes the remaining double bond to form (+)-pulegone. (+)-Pulegonereductase (PR) then reduces this double bond using NADPH to form (−)-menthone. (−)-Menthonereductase (MR) then reduces the carbonyl group using NADPH to form (−)-menthol.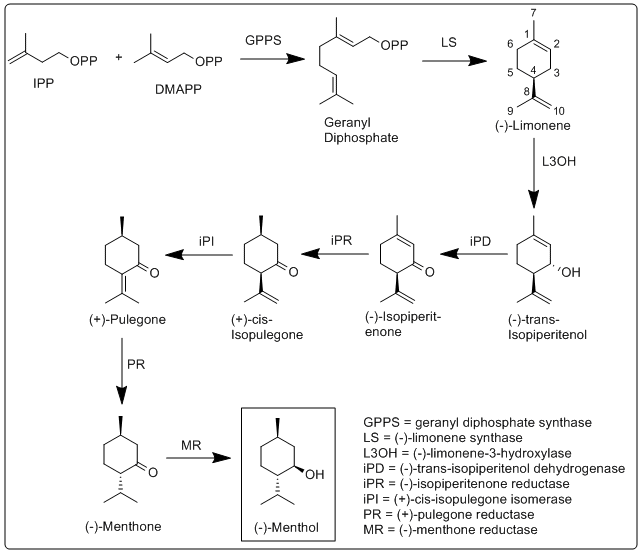
Production:
As with many widely used natural products, the demand for menthol greatly exceeds the supply from natural sources. In the case of menthol it is also interesting to note that comparative analysis of the total life-cycle costs from a sustainability perspective, has shown that production from natural sources actually results in consumption of more fossil fuel, produces more carbon dioxide effluent and has more environmental impact than either of the main synthetic production routes.
Menthol is manufactured as a single enantiomer (94% ee) on the scale of 3,000 tons per year by Takasago International Corporation. The process involves an asymmetric synthesis developed by a team led by RyōjiNoyori, who won the 2001 Nobel Prize for Chemistry in recognition of his work on this process: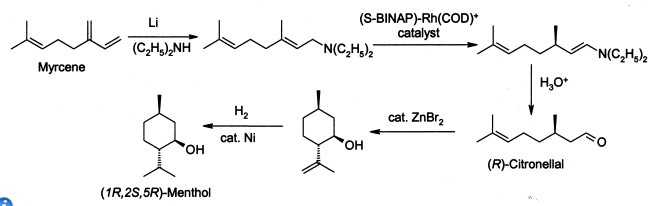
The process begins by forming an allylic amine from myrcene, which undergoes asymmetric isomerisation in the presence of a BINAP rhodium complex to give (after hydrolysis) enantiomerically pure R-citronellal. This is cyclised by a carbonyl-ene-reaction initiated by zinc bromide to isopulegol, which is then hydrogenated to give pure (1R,2S,5R)-menthol.
Another commercial process is the Haarmann-Reimer process. This process starts from m-cresol which is alkylated with propene to thymol. This compound is hydrogenated in the next step. Racemic menthol is isolated by fractional distillation. The enantiomers are separated by chiral resolution in reaction with methyl benzoate, selective crystallisation followed by hydrolysis.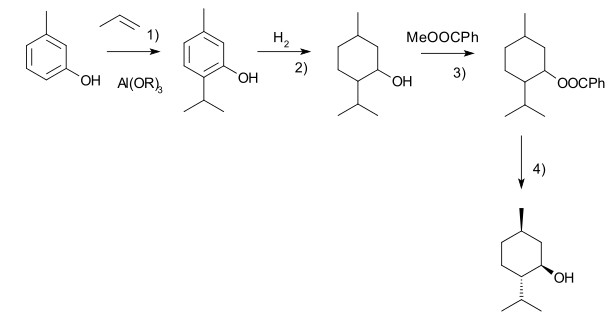
Racemic menthol can also be formed by hydrogenation of pulegone. In both cases with further processing (crystallizative entrainment resolution of the menthyl benzoate conglomerate) it is possible to concentrate the Lenantiomer, however this tends to be less efficient, although the higher processing costs may be offset by lower raw material costs. A further advantage of this process is that d-menthol becomes inexpensively available for use as a chiral auxiliary, along with the more usual l-antipode.